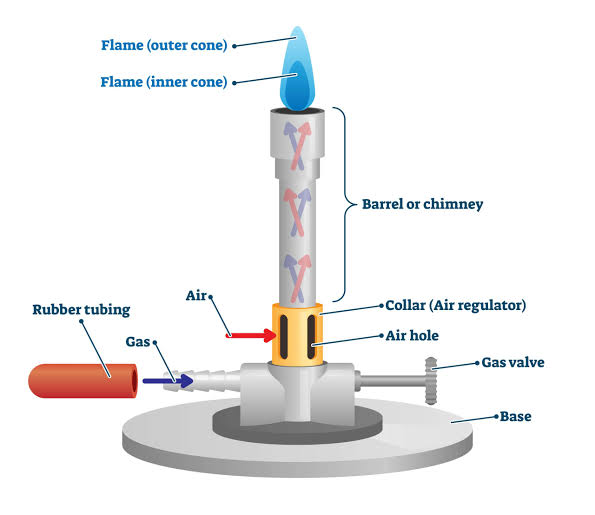
Bunsen burner is named after Robert Bunsen, a German chemist who introduced it in 1885. It is a common piece of laboratory equipment that produces a single open gas flame which is used for heating, sterilizing or combustion. The gas can be natural gas or a liquefied petroleum gas such as propane, butane or a mixture of both.
A Bunsen burner is made up entirely of metal. In order for it work optimally it consists of a barrel that is approximately five inches long, a collar, air holes, gas intake, gas valve and a stand.
The barrel of a Bunsen burner is the metal tube that screws onto the base of the burner, with small holes referred to as the intake openings in the bottom that let air into the barrel.
When natural gas and air draw into the barrel by going through the air intake opening, the gas and air mixture ignites through the top end of the barrel to make Bunsen burner functional.
Avoid touching the barrel as it can get very hot while in use and can stay hot long after it has stopped being used.
The collar of the Bunsen burner is located around the air holes on the bottom of the barrel. The main function of the collar is to control how much oxygen can enter the Bunsen burner and therefore how much oxygen can mix with the gas.
The more oxygen is allowed to enter the Bunsen burner, the higher the intensity of heat or hotness. Always light the Bunsen burner with the air holes completely covered by the collar.
The gas flow valve of the Bunsen burner is attached to the base, directly underneath with the barrel screws. The main function of the gas flow valve is allowing gas into the barrel; accordingly it can be adjusted to the counter clockwise turn to allow full gas or clockwise turn to allow less gas or turn off the Bunsen burner completely.
Gas intake/inlet tube is attached to the base and it is where the gas enters the Bunsen burner and mixes with the oxygen. The gas inlet has a short length of rubber hose attached to it. The rubber hose connects the Bunsen burner with the gas tap on your lab bench.
The air hole is located towards the bottom of the barrel and allows air to enter the Bunsen burner, where it mixes with the gas. The air hole can be partially or completely covered by turning the collar.
The base of the Bunsen burner is hexagonal or round in shape. The base is designed to be heavy and sturdy to minimize incidents of Bunsen burner tipping over.

Bunsen burner produces two types of flame that is luminous and non-luminous flame. Luminous flame is produced when the air-hole is completely or slightly closed and there is no enough amount of air entering the barrel. This flame is yellow or orange in color and also appears flickering and unsteady.
Non-luminous flame on the other hand is produced when the air hole is completely open. In this case, enough amount of oxygen/air enters the barrel and mixes with the gas producing a pale blue flame which burns steadily. The mixture of air and gas is optimally about 1 part gas to 3 parts air. The non-luminous flame is usually not clearly visible and hotter than the luminous flame. It is also easy to control. This explains why it is most preferred for heating in laboratory.
The amount of air entering the barrel of the Bunsen burner has an influence on the size of the flame and heat produced. The more the amount of oxygen entering the barrel, the larger the size of the flame and the more the amount of heat generation. However, when excess gas enters into the barrel, it can extinguish the flame.