The law of conservation of energy states that energy can neither be created nor destroyed – it transforms from one form to another. For example, solar panels do not create energy. They harness energy from the Sun and convert light energy into electrical energy. All types of energy obey this law.
As a result of energy conservation, the total energy in an isolated system is constant. If there is a loss of energy in one part of an isolated system, there must be a gain in another part.
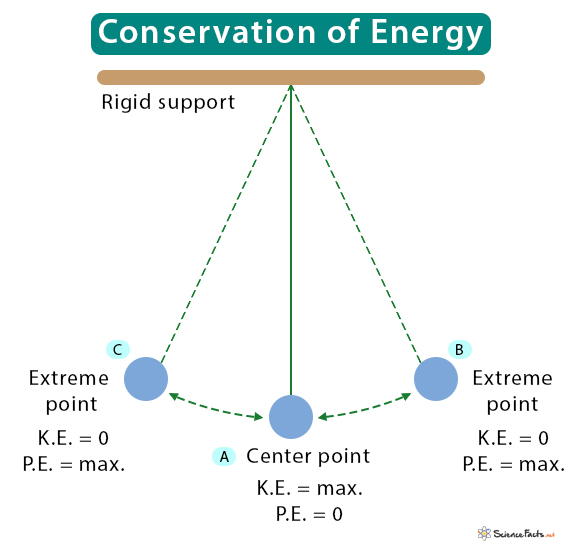
The motion of a pendulum is shown in the image below. It passes through a center point (A), reaches the extreme point (B), swings back to the center point, and finally gets to the other extreme point (C). Its kinetic energy (K.E.) is zero at the top of its swing, and its potential energy (P.E.) is maximum. In other words, when it reaches the top, all its kinetic energy is converted into potential energy.
The stored potential energy is converted into kinetic energy when the pendulum returns to the center. At the center, the potential energy is zero, and the kinetic energy is maximum. Therefore, at any time, the sum of the two is constant. Since the sum of kinetic and potential energy gives mechanical energy, a swinging pendulum is an example of the conservation of mechanical energy.

When a block slides down a ramp, its potential energy is converted into kinetic energy. However, due to friction, some energy is lost as heat. At any point, the sum of potential energy, kinetic energy, and thermal energy due to friction remains constant. The block and the ramp as a whole constitute an isolated system.
During a collision between two bodies, the sum of kinetic energy before collision equals the sum after the collision.
When a stick of dynamite explodes, the chemical energy present in the explosive is converted into kinetic energy, heat, sound, and light. The sum of all four energy forms is equal to chemical energy.
In a combustion reaction, fuel burns in the presence of oxygen to produce sound and heat. Thus, all the chemical energy present in the fuel is converted into kinetic energy, heat energy, and sound energy. If we add the three energies, the sum equals the fuel’s chemical energy.
The quantity of heat obtained by consuming food during the daily food intake of an animal equals the daily amount of energy released by the animal in the forms of work done, heat generated, and energy in the waste products. We assume that the animal is not gaining or losing any weight.
The conservation of energy law is one of the fundamental laws of all science disciplines. In several areas of science, energy conservation is applied to derive many essential equations. Let us take the example of the first law of thermodynamics to understand this concept.
Consider a system that takes in heat and does valuable work. For this system, the energy conservation equation is given by
UT: Total energy of the system
Ui: Internal energy of the system
Q: Heat added to or removed from the system
W: Work done by or on the system
Einstein’s Mass-Energy Equation
Noted physicist Albert Einstein discovered in the early 20 th century that mass could be converted into energy, known as mass-energy equivalence. The amount of energy associated with a specific amount of mass is given by the following famous equation in physics.
c: Speed of light (=3 x 10 8 m/s)
ReferencesArticle was last reviewed on Friday, February 17, 2023